Benefits of CoolSculpting Elite for Non-Surgical Fat Reduction
CoolSculpting is an FDA-cleared, non-surgical fat reduction treatment using controlled cooling that eliminates diet and exercise resistant fat.
- FDA-cleared
- Non-Invasive
- Millions of treatments performed worldwide
- Reduces fat layer by 20-24% per treatment
- Even if you work out, this treatment is perfect for diet and exercise resistant Fat

How does CoolSculpting Work?
This fat cell treatment functions on a simple biological concept. Fat cells are not designed to withstand very low temperatures, eventually killing them after a long period of exposure.
CoolSculpting uses this knowledge combined with safety and the precision of technology to effectively eliminate fat. By maintaining a controlled climate and exposure time frame, this treatment successfully kills fat cells without any surrounding tissue damage.
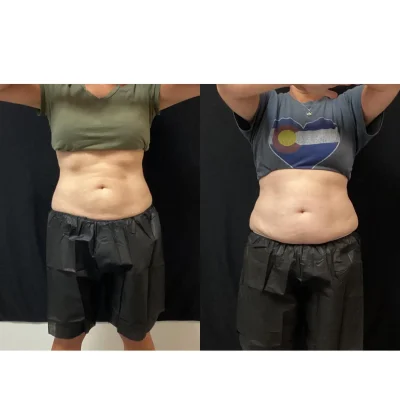
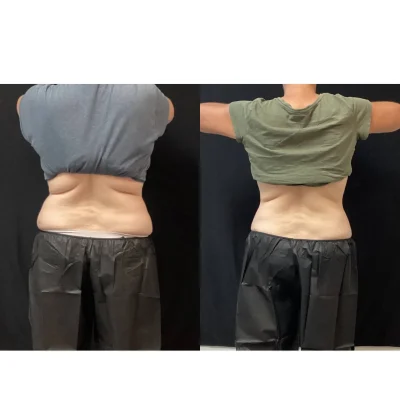
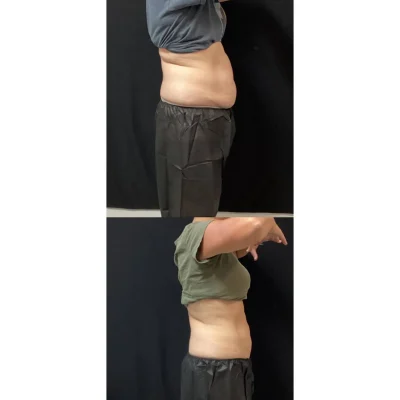
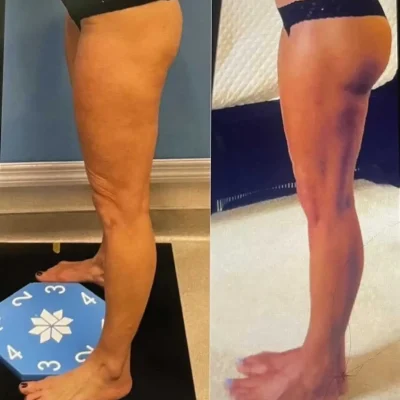
Clients and their trained team will target stubborn areas where fat can be difficult to manage. Some regions are more popular than others, such as the chin, thighs, hips, and abdomen areas.
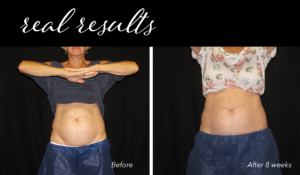
After the initial treatment, the body will work for up to 4-6 months to excrete the dead fat cells. The client will begin to see a reduction in the targeted area(s) as early as 3 weeks.
- Your body has a fixed number of fat cells that get bigger or smaller as you gain or lose weight.
- When you have the CoolSculpting treatment, fat cells freeze and start to die off.
- During the process of cell death, fat cells begin to collapse.
- Over the months that follow your procedure, other cells consume the dead fat cells, which are naturally processed and eliminated from the body.
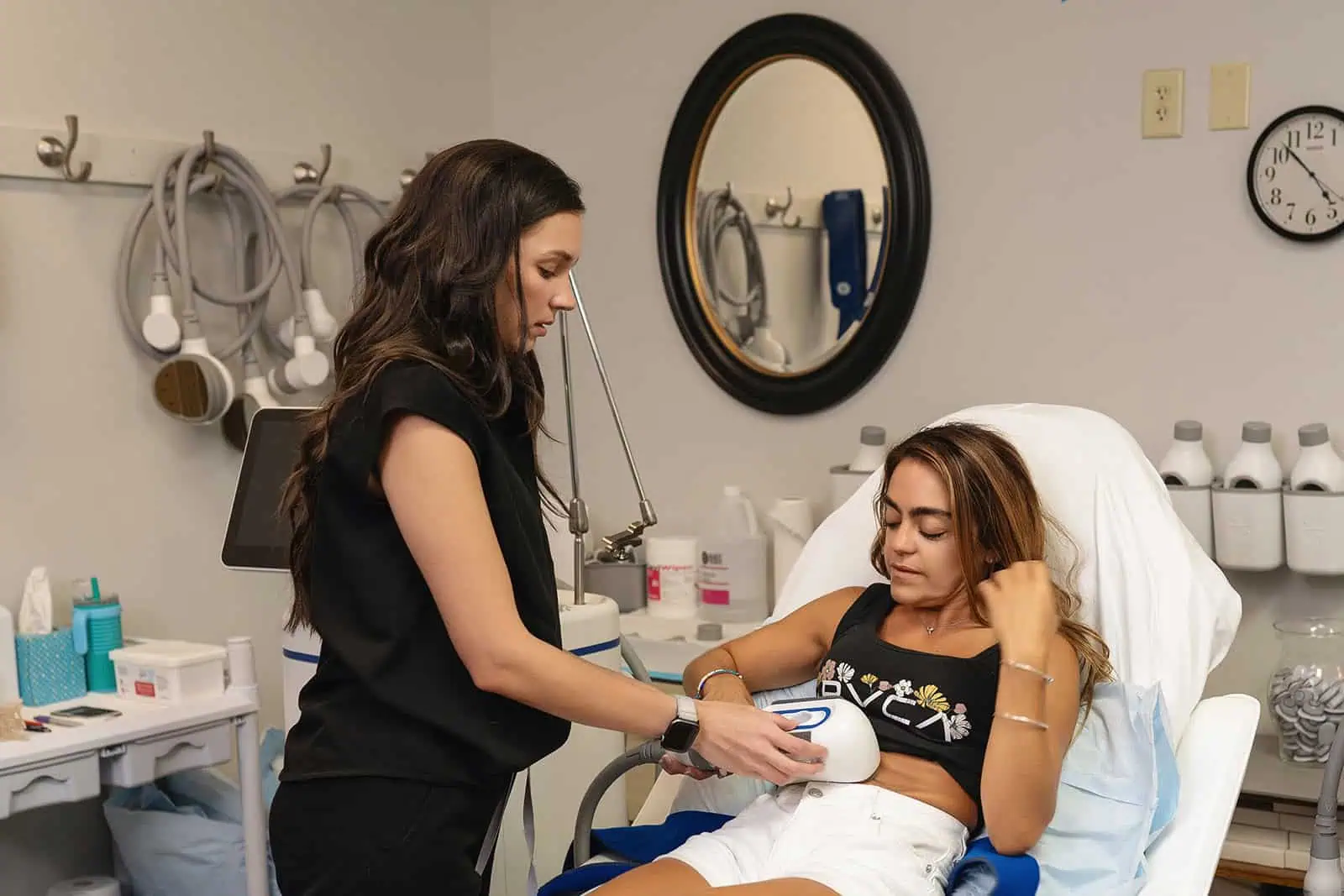


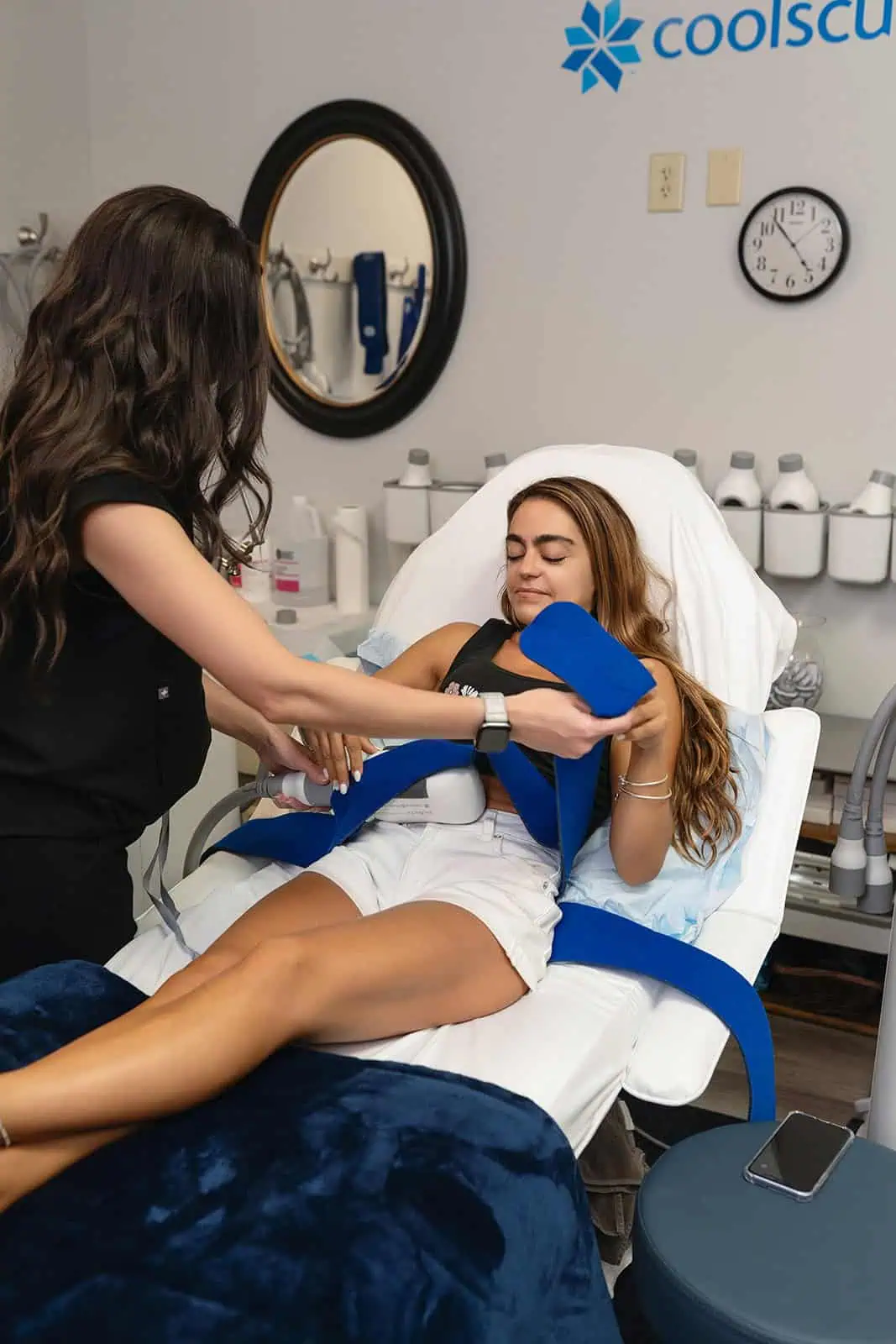









CoolSculpting® and CoolSculpting® Elite Uses
CoolSculpting® and CoolSculpting® Elite Important Safety Information
These procedures are not for everyone. You should not be treated with CoolSculpting® or CoolSculpting® Elite if you suffer from cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria.
Tell your doctor if you have any medical conditions including recent surgery, pre-existing hernia, and any known sensitivities or allergies.
During the procedure you may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching, and cramping at the treatment site. These sensations lessen as the area becomes numb. Following the procedure, typical side effects include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, or skin sensitivity, and sensation of fullness in the back of the throat after submental or submandibular area treatment.
Rare side effects may happen in 1 to 10 out of 10,000 CoolSculpting® and CoolSculpting® Elite treatments (between 0.01% to 0.1%). One such rare side effect is a visible enlargement in the treated area, which may develop 2 to 5 months after treatment, will not resolve on its own, and may require surgical intervention for correction.
Please see full Important Safety Information for CoolSculpting® and CoolSculpting® Elite on CoolSculpting.com.
Patient Results May Vary. CoolTone® Uses
CoolTone® Important Safety Information
The CoolTone® procedure is not for everyone. You should not have the CoolTone® treatment in areas with metal, electrical, or electronic implants/devices like cardiac pacemakers, implanted hearing devices, implanted defibrillators, implanted neurostimulators, drug pumps, or hearing aids.
Tell your doctor if you have any medical conditions as CoolTone® should not be used over a menstruating uterus, over areas of the skin that lack normal sensation, in patients with fever, malignant tumor, hemorrhagic conditions, epilepsy, recent surgical procedure, pulmonary insufficiency, or pregnancy.
CoolTone® should be used with caution in patients with Graves’ disease (an autoimmune disorder that causes overactive thyroid), active bleeding disorders, or seizure disorders.
Women who are close to menstruation may find that it comes sooner, or cramping is increased or intensified with CoolTone® treatments, therefore, it is recommended to not undergo treatment during this time of the month.
CoolTone® should not be used in the heart or head areas, areas of new bone growth, over the carotid sinus nerves, or over the neck or mouth.
CoolTone® should not be applied over swollen, infected, inflamed areas or skin eruptions. Caution should be used for patients with suspected or diagnosed heart problems.
Common side effects may include, but may not be limited to, muscular pain, temporary muscle spasm, temporary joint or tendon pain, and redness at or near the treatment site.
Ask your Healthcare Provider if CoolTone® is right for you.
Please see full Important Safety Information for additional information at coolsculpting.com/cooltone.
Patient Results May Vary.
CoolSculpting Elite Before and After Results